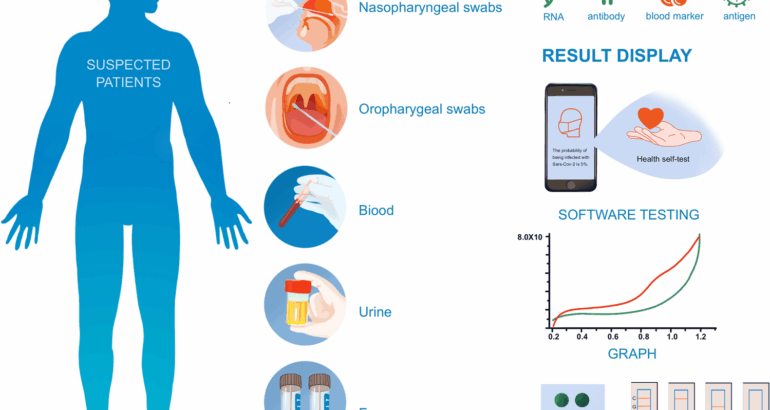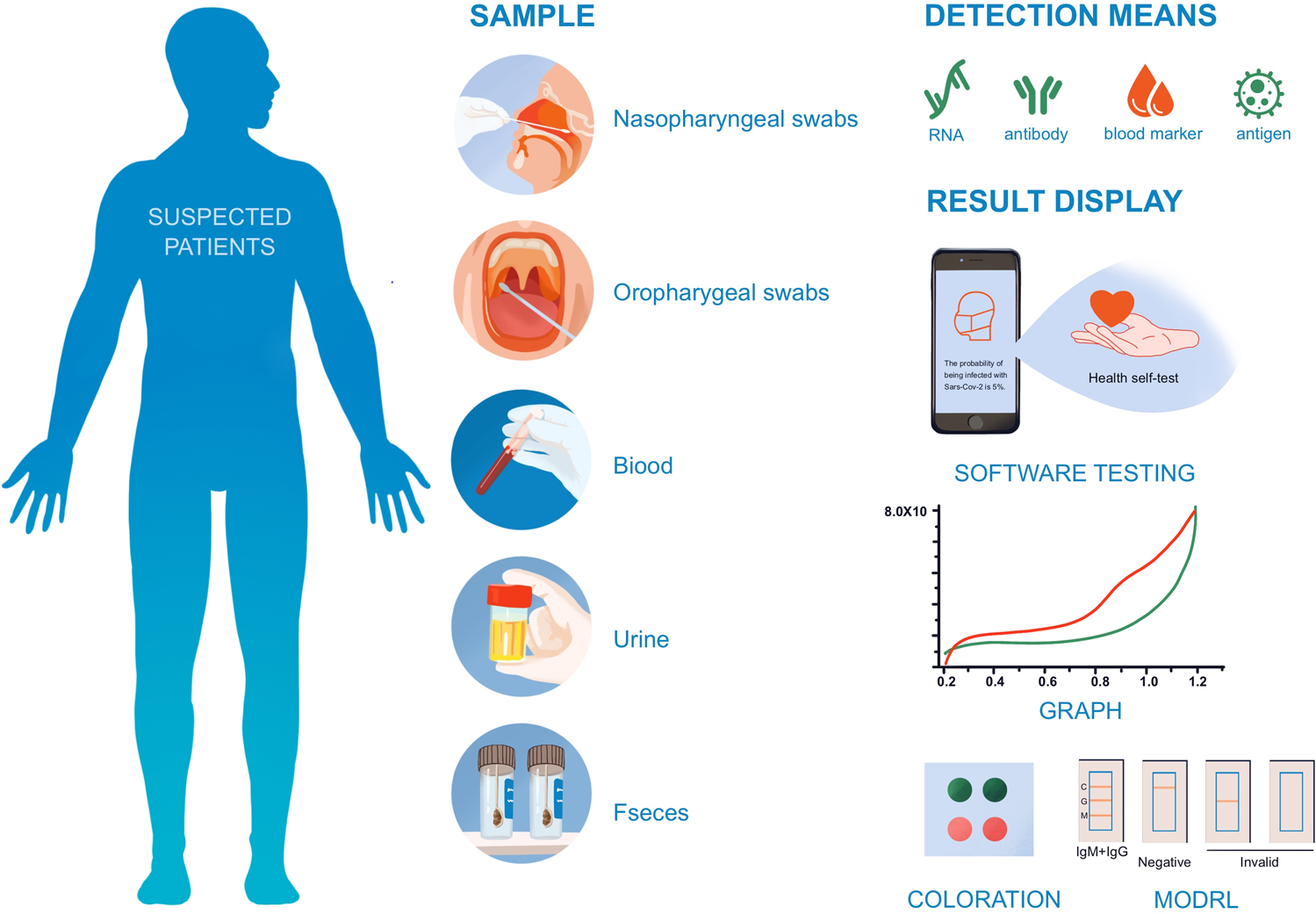
For sponsors developing rapid infectious disease diagnostics, the landscape is shifting fast. Rapid diagnostics provide results in minutes, whereas conventional laboratory tests take hours or days. This shift creates a significant challenge: clinical trial strategies designed for slow, centralized tests won’t work for fast, decentralized ones. This isn’t just a technology upgrade. It’s a fundamental shift that requires new clinical trial strategies tailored for rapid, decentralized diagnostics.
The Clinical Imperative: The Importance of “Under 2 Hours” The World Health Organization’s (WHO) declaration of mpox as a public health emergency reinforced a now-familiar truth: conventional diagnostic infrastructure often fails under the demands of outbreak conditions. The COVID-19 pandemic exposed critical gaps in testing capacity, turnaround times, and real-time clinical decision support. As a result, infectious disease diagnostics are moving toward decentralized, quick solutions that can provide actionable results at or close to the point of care. Rapid diagnostic tests with turnaround times under two hours are not merely technological improvements—they represent a fundamental shift in clinical utility. These tools are designed to support decisions that must be made within tight windows, particularly in emergency departments, urgent care, and outbreak response settings. In many cases, the timing of a result directly impacts patient isolation protocols, treatment initiation, and antimicrobial stewardship efforts.
However, clinical trial methodologies have not always kept pace with this shift. Traditional trial designs emphasize analytical performance under controlled conditions, but rapid diagnostics require a broader validation approach. It is no longer sufficient to demonstrate sensitivity and specificity alone. Studies must also assess how diagnostic speed translates into clinical action: whether faster diagnosis leads to more timely treatment, earlier infection control measures, or reductions in unnecessary antibiotic use. Validating rapid diagnostics, therefore, requires trial designs that reflect the real-world settings in which these tools are deployed and the clinical decisions they are intended to support.
Overcoming Obstacles in the Real World Bridging the Infrastructure and Environment Gaps
Currently, most molecular assays for infectious disease testing remain confined to academic medical centers or commercial laboratories, where specialized equipment and trained personnel are readily available. The high cost of primary equipment for portable molecular platforms creates significant barriers to accessibility and widespread adoption, particularly in decentralized or resource-limited settings.
A notable example is the Dragonfly platform, a portable molecular diagnostic tool developed for mpox testing. This platform demonstrated impressive performance: 96.1% sensitivity and 100% specificity for orthopoxviruses using 164 clinical samples. Yet, its true innovation lies in its adaptability across diverse clinical environments. To address cost constraints, the researchers developed a simple isothermal heater manufacturable for under £100, eliminated the need for cold-chain storage, and incorporated a companion app to facilitate result interpretation.
This case exemplifies the critical need to bridge the implementation gap: clinical trials must demonstrate not only that a diagnostic performs well analytically, but also that it functions effectively in the hands of non-laboratory personnel, under suboptimal conditions, and with variable sample handling quality. To validate rapid diagnostics in real-world conditions, trial designs must go beyond analytical accuracy under ideal lab settings.
They need to account for the following operational and environmental factors:
Site capability: Not all healthcare facilities have experience with POC testing or the staff trained to administer rapid molecular diagnostics. Selecting trial sites that reflect the intended deployment environment, such as clinics with established decentralized testing protocols and flexible workflows, is critical.
Environmental stressors: Temperature fluctuations, humidity, inconsistent storage, and variable sample handling quality can impact test performance. To demonstrate robustness, trials must mimic these conditions.
Operational workflow: The test must fit into fast-paced clinical encounters, often within tight time windows. Usability and integration with existing processes are key to successful adoption.
Training and competency: Many rapid diagnostics are administered by healthcare workers with minimal lab experience. Error rates under real-world conditions and whether users can correctly complete the test with the provided training should be evaluated in trials. Combining these factors into a comprehensive validation strategy ensures that rapid diagnostics are not only analytically sound but also operationally feasible and scalable in the settings where they are most needed. By bridging this infrastructure and environment gap, clinical trials generate evidence that supports regulatory approval, payer acceptance, and, ultimately, patient benefit.
Usability and Human Factors: Evaluating the End-User Experience
In decentralized and point-of-care settings, diagnostic tests are often administered by individuals with minimal laboratory training, such as nurses, community health workers, or patients themselves. Even highly accurate tests can underperform in these environments if they are not intuitive or robust to user error.
Formal assessments of usability and human factors should be included in clinical trials to guarantee success beyond analytical validation. Key considerations include:
Instruction adherence: Whether intended users can follow test protocols without supervision.
Result clarity: Whether results, visual or digital, are easy to interpret under varying conditions.
Error identification and mitigation: Common failure modes during sample handling or result entry, and whether they can be reduced through better design or training.
Workflow fit: Whether the test can be run and results acted upon without disrupting patient flow or care protocols.
These factors often determine whether a diagnostic can be adopted at scale. Usability data, particularly when gathered across diverse user profiles, strengthens both regulatory submissions and commercial readiness.
Tackling Multiplex Complexity in Trial Design
There are currently several molecular multiplex syndromic panels available in the U.S.: bloodstream infections, respiratory tract infections, gastrointestinal infections, and meningitis/encephalitis. These panels bring efficiency, but clinical complexity must also be considered when designing a clinical trial.
A respiratory panel might show simultaneous positives for influenza, RSV, and rhinovirus. When interpreting multiplex results, clinicians must consider prolonged shedding periods, multiple positive results or co-infections, detection of asymptomatic carriage, and variable accuracy for different agents on the panel.
This complexity has real implications for trial design. It’s no longer enough to prove your test can detect multiple pathogens accurately. It must be demonstrated that results can guide clinical decisions. These clinical endpoints have to be taken into account: Primary endpoints that can handle multiple simultaneous positive results: Multiplex testing breaks down traditional binary positive/negative frameworks. Clinical utility assessments that evaluate decision-making with complex result patterns: How do clinicians interpret and act on multiplex results, and does that action lead to better outcomes?
Post-market surveillance built into trial design from day one: Real-world complexity emerges after deployment
Demonstrating Value: Cost-Effectiveness and Regulatory Expectations
Closing the Cost-Effectiveness Evidence Gap
Demonstrating cost-effectiveness is essential for the adoption of rapid infectious disease diagnostics, particularly when these tools carry higher per-test costs or require changes to standard care pathways. Payers, health systems, and regulatory bodies increasingly expect evidence that a new diagnostic not only improves clinical outcomes but also delivers measurable value across the healthcare continuum.
To meet these expectations, health economic endpoints should be integrated into clinical trial design from the outset.
The following are key evaluation areas:
Time to appropriate therapy: Measuring whether earlier diagnostic results translate into faster, more targeted treatment decisions.
Healthcare resource use: Assessing impacts on emergency department throughput, hospital admissions, and length of stay.
Antimicrobial stewardship: Evaluating the test’s ability to reduce unnecessary or inappropriate antibiotic prescriptions.
Downstream utilization: Capturing broader effects on repeat visits, follow-up testing, or escalation of care.
These data help establish a diagnostic’s role not only in clinical decision-making but also in improving system-level efficiency. Embedding health economic measures in trial protocols ensures that performance claims are supported by real-world value evidence, which can accelerate both regulatory clearance and market access.
Rapid Infectious Disease Diagnostics and the Changing Expectations of Regulatory Agencies Regulatory agencies have demonstrated increasing flexibility and responsiveness in evaluating POC and rapid diagnostic technologies. Recent approvals reflect a growing recognition of the clinical value these tools provide, especially in addressing urgent public health needs.